Fertilizers
A soil amendment could be a fertilizer or any composted organic matter which improves the texture of the soil without adding additional chemicals.
A fertilizer is any product added to the soil which increases the soil’s chemical content and which has the ability to improve or maintain plant growth.
Fertilizers are available in natural or chemical forms. Natural fertilizers are derived from dead organisms or their waste products. These include such things as fish emulsion, animal manures, meals made from the bones, blood, or feathers of animals or from alfalfa, cottonseeds or soybeans. Most natural fertilizers contain lower levels of nutrients than chemical products. They release their nutrients more slowly than chemical fertilizers because they do not dissolve in water and must be broken down by soil microbes before they can be absorbed by plants.
Chemical fertilizers are derived from the chemical sources listed on the product label. These sources are frequently minerals dug from the earth and then blended to produce the desired ratio of plant nutrients. Compared to natural fertilizers, they usually provide higher levels of nutrients and are faster acting; they typically cost less too.
Every fertilizer label states the percentage by weight of the three macronutrients in the following order; Nitrogen (N), Phosphorus (P) and Potassium (K). A fertilizer labeled 12-8-4 contains 12% Nitrogen, 8% Phosphorus, and 4% Potassium. On the back side of all Master Nursery fertilizers will also be listed the percentages of secondary nutrients; Calcium (Ca), Magnesium (Mg) and Sulfur (S) as well as micronutrients Zinc (Zn), Manganese (Mn), and Iron (Fe). Animal manures are an excellent source of organic matter which can be used to amend deficient soils. They should not be considered fertilizers, however. The N, P, K of animal manures is very low (Poultry 2-1-1; Steer 1-0-1; Horse 1-0-0) and plants relying only on animal manures as fertilizers soon develop nutrient deficiencies.
Fertilizers are sold as liquids or solids. Liquids including Master Nursery® Fish Emulsion, Master Nursery® Liquid Gold, and water soluble crystals deliver nutrients to the roots immediately. They must be reapplied frequently because their nutrients leach through the root zone quickly. Solid fertilizers are usually sold as powders, granules or pellets. They may be spread on the surface of the soil or scratched in with a cultivator. These solid fertilizers take longer to dissolve and may continue to work for a month or longer. Other solids include controlled release fertilizers sold as tablets, spikes or bead-like granules (Osmocote®) and release nutrients gradually for 3 to 9 months. Osmocote® contains only N, P and K as do most of the tablets and spikes. Additionally, the tablets and spikes when placed into the soil concentrate their nutrients in a very narrow area. Recently, Osmocote® has reformulated their product to be called Osmocote® Plus which contains N, P, K plus numerous of the secondary and micronutrients.
Plant growth requires about 12 or 13 elements in measurable amounts (Carbon-C, Hydrogen-H, Oxygen-O, Nitrogen-N, Phosphorus-P, Potassium-K, Iodine-I, Sulfur-S, Calcium-Ca, Iron-Fe, and several in only trace amounts. C, H, O and N make up about 98% of a plant’s structure.
Carbon (C), Hydrogen (H) and Oxygen (O): These three elements are never in short supply for the plant because they are provided by CO2 in the air and by water (H2O) in the soil. C, H and O are used by the plant to produce carbohydrates and oils in the form of sugars, starches, cellulose (wood) and various oils (olive, palm, corn, canola, etc.). All of these compounds are composed of only three elements, none of which we ever need to add as fertilizer.
The soil nutrients used by plants have many roles and it is not possible to assign a single function to each element.
Nitrogen (N): Nitrogen is the element most critical in a fertilizer because it is easily flushed from the soil and is required for the production of proteins. Remember, plants cannot absorb manufactured foods (proteins, sugars, oils, etc.) They must make whatever they need from the elements available in the soil. Every single plant cell has protein as part of its structure. In the cells are specialized proteins called enzymes which regulate every reaction within the cell and between cells. If there were no enzymes or not the right ones, life in the cells would stop. So the bottom line is; no nitrogen means no protein means no cell parts and no life reactions, which equals a dead plant. The plant does not die all at once, it warns you by wilting, turning yellow then brown, then dead.
If you remember your science class; air is about 75% nitrogen so why don’t plants use that to meet their needs? Unfortunately, it’s the wrong kind of nitrogen. Only a few kinds of microbes are able to use the nitrogen in the air and they change a small amount to a form usable by plants. When you turn your bag of 25-3-8 fertilizer over and look at the ingredients you see ammoniacal nitrogen 5%, urea nitrogen 14%, nitrate nitrogen 5%, Water Insoluble organic nitrogen 1%. These different forms of nitrogen are not equally available to plants. Plants can use only nitrate nitrogen and so the other forms must be changed to nitrates by microbes in the soil. Ammoniacal is most quickly changed, Urea second most quickly and the water insoluble takes up to a month. Such a mixture gives the plant a fast boost (nitrate) and then spreads the others out over a longer period. If the plant lacks nitrogen and is given nitrogen fertilizer, we see a general improvement in the plant. More and greener leaves, more twigs and branches and perhaps new flower buds.
Phosphorus (P): In the Bay Area, phosphorus deficiency is seldom a problem. It is not very soluble and does not leach easily. It remains where it is placed and we are told to place some near the roots when plants are transplanted. Phosphorus is part of the molecule (ATP) used by plants to provide energy for all of its cells including: growth, reproduction, movement, flowering, food production, chemical conversions such as carbohydrates to oil and so on. It is also part of the DNA molecule which controls all of the plant’s activities. Many of these activities are most apparent in the roots and flowers of plants and so phosphorus is often credited with stimulating roots and flower growth. A few plants (especially tomatoes and corn) will signal a phosphorus deficiency by having the underside of leaves turn purple.
Potassium (K): Potassium is also relatively insoluble and so leaching and soil deficiencies in the Bay Area are rare. Potassium does not become part of the plant’s structure. Its function is in solution as an activator for cell enzymes. Without it, all of the plant cell’s activities which require enzymes would stop and the cells (leaves, flowers, roots, stems) would die. As with phosphorus, potassium is associated with root and flower development and is often recommended to stimulate their growth.
Secondary Nutrients and Micronutrients (Ca, Mg, S, Zn, Mn, and Fe): The secondary nutrients and micronutrients make up about 0.8% of a plant’s parts. Each has numerous functions without which the plant would show deficiency symptoms. Calcium (Ca) is part of the structure of every cell wall in the plant and also helps control water movement in the plant. Magnesium (Mg) is the central part of the chlorophyll molecule which produces the green color in plants. Chlorophyll is the magic molecule which captures energy from the sun and enables plants to produce sugars, starches, fats, oils, proteins and even the color in roses. Without chlorophyll, there would be no life on Earth as we know it. Iron (Fe) is not part of the chlorophyll molecule but is necessary for its production. This accounts for the loss of green coloring and yellowing of plant leaves suffering from an iron deficiency. Sulfur (S) is needed for plants to produce protein
molecules. (See previous page.) Sulfur is also responsible for adjusting pH within and outside the plant cells.
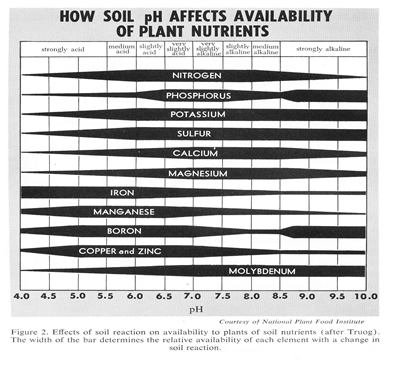
pH is a term used by chemists to measure the acidity or alkalinity of a soil on a scale of 0 to 14. Seven (7) is neutral; below 7 is acidic and above 7 is alkaline. Most plants grow best in a pH of 6.5 or lower (slightly acidic). Blueberries need a soil with a pH of 4.5 to 5.5. Many minerals in the soil will not dissolve unless the soil is acidic. The acid (low pH) in the soil dissolves the minerals so the plants can absorb them. The clay soil in the Bay Area is alkaline (7.4 or greater) and so many minerals (Fe, P, K, etc.) may be present but do not dissolve and so are not available to plants. Adding more Iron will not get rid of the yellow in plant leaves because the Iron does not dissolve. To correct the problem, add sulfur or iron sulfate to the soil to lower the pH below 7.0 so the minerals will dissolve. All of Master Nursery fertilizers contain iron and sulfur to help lower the soil and water pH into a more favorable range.
Understanding fertilizers and pH is particularly important when growing fruits and vegetables. Landscape plants and trees drop much of their dead foliage and twigs on the ground which becomes part of the soil and replaces minerals taken from the ground. When we grow fruits and vegetables, we are constantly taking minerals away each time we harvest a crop. Consequently, orchards and vegetable gardens are the first to show nutrient deficiencies which require fertilizing and pH adjustment